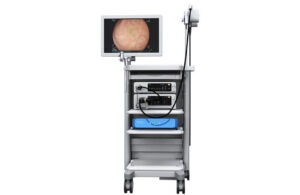
Medtronic wins CE mark for next-gen GI Genius module, ColonPRO software
Medtronic (NYSE: MDT)+ announced today that it received CE mark for its ColonPRO fourth-generation software for AI-assisted colonoscopy.

Medtronic (NYSE: MDT)+ announced today that it received CE mark for its ColonPRO fourth-generation software for AI-assisted colonoscopy.
Biotronik announced today that it received FDA approval for its Solia CSP S pacing lead for left bundle branch area pacing (LBBAP).

Allurion Technologies (NYSE:ALUR) announced today that the FDA granted premarket approval (PMA) for its Allurion Gastric Balloon System.

Medtronic is investing in the rollout of the Symplicity Spyral renal denervation procedure, including hiring for new market development roles, CEO Geoff Martha said on an earnings call.
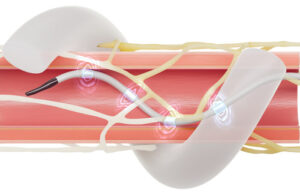
Medtronic continues to expand the growth opportunities for its hypertension-treating Symplicity Spyral renal denervation system.
GE HealthCare (Nasdaq: GEHC)+ announced today that it received FDA clearance for a trio of new MRI offerings.

LOUISVILLE, Ky., Feb. 19, 2026 /PRNewswire/ — Breath Diagnostics, Inc., a leader in breath-based molecular diagnostics powered by patented microreactor capture technology, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to its OneBreath™ platform.

DAYTON, Ohio, Feb. 19, 2026 /PRNewswire/ — Innovative Sterilization Technologies (IST) announced today that the ONE TRAY® sterilization container has received a second clearance from the U.S. Food and Drug Administration (FDA), significantly expanding its use across hospital and surgical facility workflows.

CHICAGO, Feb. 19, 2026 /PRNewswire/ — Sibel Health, a leader in medical-grade wearable sensor technology, today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s recent Letter of Intent (LOI) into the Clinical Outcome Assessment (COA) Qualification Program under the Drug Development Tool (DDT) framework. The acceptance marks a significant milestone in advancing objective cough frequency measurement for adult patients with chronic refractory cough (CRC) using a novel Cough Monitoring sensor, the Aria sensor.
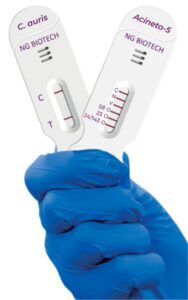
NG Biotech manufactured the assays in France, with Hardy Diagnostics serving as the exclusive distributor in the US.