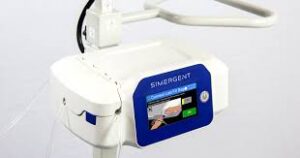
Simergent Announces FDA Clearance of the Archimedes™ Home Dialysis System
OKLAHOMA CITY and CHICAGO, Oct. 14, 2025 /PRNewswire/ — Simergent, a privately held company dedicated to advancing treatment for patients with kidney failure, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the company’s Archimedes™ automated peritoneal dialysis (APD) system for the treatment of patients with End Stage Kidney Disease (ESKD) in clinical and home settings.