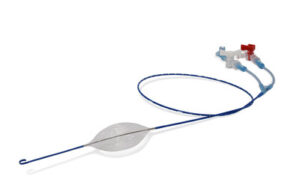
Prytime Medical Devices Receives World’s First Extended Duration FDA 510(k) Clearance for pREBOA-PRO™ Catheter
BOERNE, Texas, Oct. 8, 2025 /PRNewswire/ — Building once again on their first mover position as a leader in endovascular hemorrhage control and resuscitation, Prytime Medical Devices, Inc. (Prytime) this week announced a new FDA 510(k) clearance for their flagship pREBOA-PRO™ Catheter.