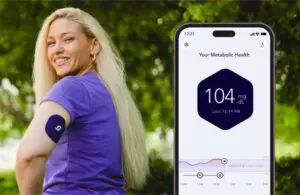
Signos gets FDA green light for over-the-counter CGM for weight management
Signos today announced a major step forward in bringing over-the-counter (OTC) continuous glucose monitoring (CGM) to market.

Signos today announced a major step forward in bringing over-the-counter (OTC) continuous glucose monitoring (CGM) to market.

Microbot Medical (Nasdaq:MBOT) announced today that it received a new patent covering a modular robotic surgical system.
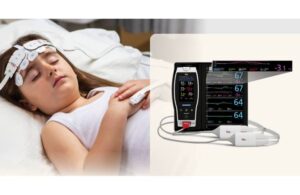
Masimo (Nasdaq: MASI)+
announced today that it received FDA 510(k) clearance for expanded indications for its O3 regional oximetry technology
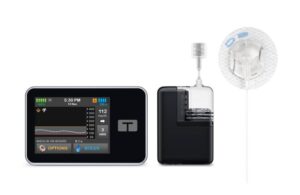
Tandem Diabetes Care (Nasdaq:TNDM) has a new infusion set on the way to pair with its insulin delivery offerings.

ANKARA, Türkiye, Aug. 18, 2025 /PRNewswire/ — Nerveblox, an AI software solution by SmartAlpha, designed to assist physicians in using ultrasound while administering regional anesthesia procedures commonly known as ‘nerve blocks’, has received U.S. Food and Drug Administration (FDA) 510(k) clearance.
The company is collaborating with local companies and healthcare providers to distribute the ulcer test.
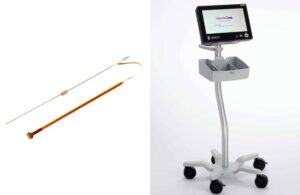
NeuroOne (Nasdaq:NMTC) announced today that it received FDA clearance for its OneRF trigeminal nerve ablation system.

Onward Medical announced today that it received FDA investigational device exemption (IDE) for its ARC-IM system.

The test was previously granted a breakthrough device designation by the US regulator.

DeepSight Technology announced today that it received FDA 510(k) clearance for its NeedleVue LC1 ultrasound system