Interventional Systems, HICREN get regulatory nod for mini spine surgical robot in China
Interventional Systems and HICREN today announced approval for their joint venture’s surgical robot platform in China.

Interventional Systems and HICREN today announced approval for their joint venture’s surgical robot platform in China.
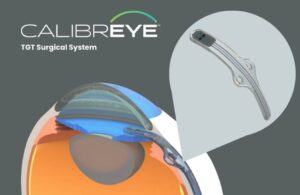
Myra Vision announced today that it received FDA investigational device exemption (IDE) approval for its Calibreye system
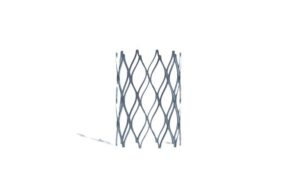
Renata Medical announced today that CMS granted a New Technology Add-on Payment (NTAP) for its Minima stent system.

The system is compatible with 1.5T and 3T magnetic resonance imaging scans and eliminates the need for an implanted battery.
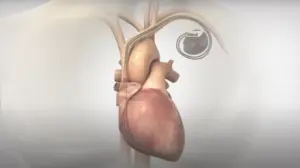
Orchestra BioMed (Nasdaq:OBIO) announced today that it began the rollout of a protocol update for its BACKBEAT study.

Nyxoah (Nasdaq:NYXH) announced today that it received FDA approval for its Genio neuromodulation device for treating sleep apnea.

SurgiBox, a medical technology company committed to improving access to safe, clean surgery at the point of need, announced today it has received the CE Mark under MDR for the medical devices that comprise its flagship product, the SurgiField System.

BEDFORD, Mass., Aug. 7, 2025 /PRNewswire/ — Instylla, Inc., a privately held company developing novel resorbable embolics for peripheral vascular embolization, announced premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for the company’s flagship product Embrace™ Hydrogel Embolic System. Embrace HES has been approved for the embolization of hypervascular tumors in peripheral arteries ≤ 5mm.
The diagnostic performance of HyCoSy with SF6 microbubbles claims to have been validated by a meta-analysis of 24 trials.
BiVacor announced today that the FDA accepted its Total Artificial Heart (TAH) into its Total Product LifeCycle Advisory Program (TAP).