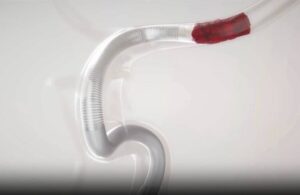
Imperative Care wins FDA nod for new Zoom 7X catheter
Imperative Care announced today that it received FDA 510(k) clearance for its novel Zoom 7X catheter for aspiration thrombectomy procedures.

Imperative Care announced today that it received FDA 510(k) clearance for its novel Zoom 7X catheter for aspiration thrombectomy procedures.

The CE mark also allows Medtronic’s automated insulin delivery system to be used during pregnancy and by children as young as 2.

TUSTIN, Calif., July 22, 2025 /PRNewswire/ — Celluma Light Therapy, the global leader in award-winning, clinically backed LED technology, announces the launch of the Celluma NOVA, the first-of-its-kind battery-powered, shape-taking LED panel with five FDA-cleared treatment modes. The Celluma NOVA treats aging skin, pain, hair restoration, body contouring, and acne — all in a single compact, rechargeable device.
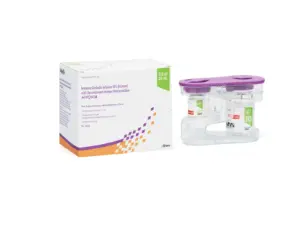
New devices designed to streamline and personalize HYQVIA® administration for patients receiving subcutaneous immune globulin therapy
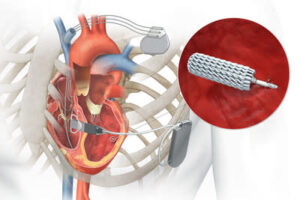
AUSTIN, Texas, July 21, 2025 /PRNewswire/ — Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center recently became the first in the nation to implant an FDA-approved novel leadless system that provides cardiac resynchronization therapy to patients with heart failure. Cardiac resynchronization therapy improves the timing of the heart’s contractions, helping to restore the normal rhythm of the heartbeat. The first procedure was recently performed by Robert Canby, M.D., cardiac electrophysiologist at TCAI.

Spirair announced today that the FDA granted 510(k) clearance for its TurbAlign bioabsorbable implant device.

Boston Scientific (NYSE: BSX)+
has received expanded FDA approval for the use of its Watchman FLX and Watchman FLX Pro devices.

NDA for TAR-200 targets high-risk non-muscle invasive bladder cancer, highlighting potential to transform treatment for patients unresponsive to BCG therapy

UroMems announced that it received FDA investigational device exemption (IDE) approval to begin a first-of-its-kind trial for its smart implant.

MINNEAPOLIS, July 17, 2025 /PRNewswire/ — Saluda Medical, Inc., a pioneer in the development and commercialization of a novel neuromodulation platform designed to transform the lives of patients with chronic neurological conditions, today announced the full commercial launch of EVA, its next-generation sensing technology, in the United States (U.S.). EVA received U.S. Food & Drug Administration (FDA) approval in December 2024 and is compatible with all commercially implanted Evoke® SmartLoop™ System patients.