Nitinotes Announces FDA IDE Approval to Initiate Pivotal U.S. Trial of the EndoZip™ System for Endoscopic Sleeve Gastroplasty
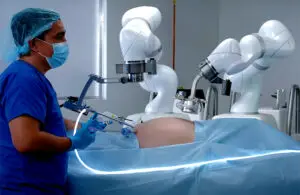
CAESAREA, Israel, June 24, 2025 /PRNewswire/ — Nitinotes, developer of the EndoZip™ System, a fully automated suturing platform for endoscopic sleeve gastroplasty (ESG), today announced it has received approval from the U.S. Food and Drug Administration (FDA) to initiate a pivotal clinical trial under an Investigational Device Exemption (IDE).