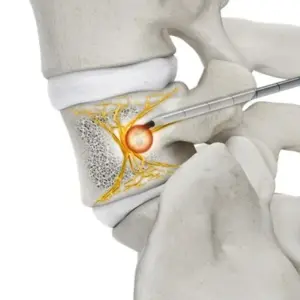
Stryker Secures FDA Clearance for OptaBlate® BVN System
New basivertebral nerve ablation device aims to relieve chronic vertebrogenic low back pain

New basivertebral nerve ablation device aims to relieve chronic vertebrogenic low back pain

Gradient Denervation Technologies announced today that it received FDA breakthrough device designation for its denervation system.

Trax Surgical today announced it received FDA 510(k) clearance to market its Linkt Compression Staple System.

Aevice Health announced today that it received FDA 510(k) clearance for its AeviceMD smart wearable stethoscope device.

ATLANTA, May 13, 2025 /PRNewswire/ — KNoW Biological has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA). This designation is an FDA program designed to expedite the review of devices and medications for serious or life-threatening conditions where there’s preliminary evidence suggesting substantial improvement over existing therapies on the market.

A patient-first solution redefines comfort and accuracy in sleep disorder testing across the U.S.

Align Technology’s non-invasive orthodontic innovation set to transform pediatric care in China.
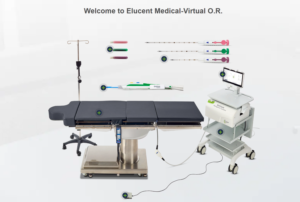
Elucent Medical announced today that it received FDA breakthrough device designation for its EnVisio X1 In-Body Spatial Intelligence system.
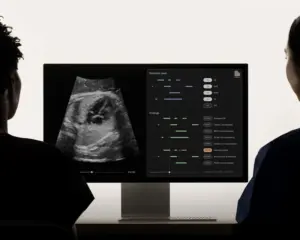
First-of-its-kind platform offers real-time fetal heart documentation and congenital heart disease detection, revolutionizing prenatal care and diagnosis.

Go-Pen ApS announced recently that it received FDA 510(k) clearance for its Go-Pen cost-effective, user-filled insulin pen.