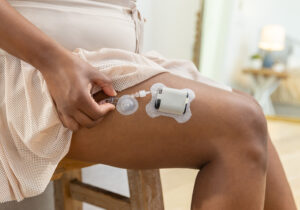
Tandem Diabetes Care subsidiary earns new FDA clearance for insulin infusion set
A subsidiary of Tandem Diabetes Care (Nasdaq:TNDM) recently picked up FDA clearance for infusion technology to support insulin delivery

A subsidiary of Tandem Diabetes Care (Nasdaq:TNDM) recently picked up FDA clearance for infusion technology to support insulin delivery

High-Precision Type 5 Indicator Enhances Patient Safety by Validating Steam Sterilization Cycles in Clinical Settings
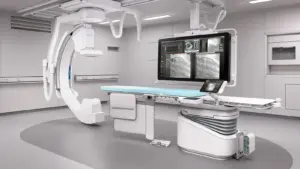
HOUSTON, May 12, 2025 /PRNewswire/ — United Imaging, a global manufacturer of modern medical imaging technology, announces that its first Interventional X-ray system is now FDA cleared. uAngio AVIVA is an intuitively bionic ceiling-mounted system that uses intelligent robotics, voice control, vision and imaging to serve as a critically important assistant to clinical staff in the interventional suite.

Zynex (Nasdaq:NYXI) announced today that it submitted its NiCO non-invasive co-oximeter device for FDA 510(k) clearance.

Milestone Approvals Enable European and UK Market Access for AI-Based Cardiac Arrest Prediction Tool

Women can now collect their own sample from the privacy of home, no speculum required, and mail it to a certified lab to be tested on the same test as the doctor’s office, with the same accuracy.
Final approval clears the way for Zydus to market Glatiramer Acetate, expanding access to a key treatment for multiple sclerosis patients in the U.S.
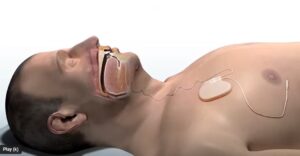
LivaNova (Nasdaq: LIVN)+
today announced 12-month data from its OSPREY study supporting an FDA submission of the aura6000 system.
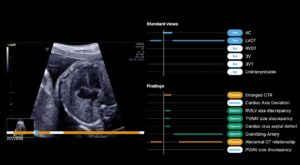
BrightHeart earned its second FDA 510(k) clearance for updates to its BrightHeart platform, an AI-powered digital screening tool for congenital heart defects (CHDs).
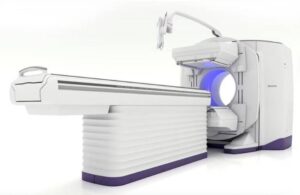
GE HealthCare (Nasdaq: GEHC) announced today that it received FDA 510(k) clearance for its Aurora nuclear medicine system and Clarify DL