BVI Medical wins FDA nod for laser endoscopy ophthalmic system
BVI Medical announced that it received FDA 510(k) clearance for its Leos (laser endoscopy ophthalmic system) for treating glaucoma.

BVI Medical announced that it received FDA 510(k) clearance for its Leos (laser endoscopy ophthalmic system) for treating glaucoma.

New implants aim to enhance outcomes in foot and ankle surgeries through advanced material technology.

Precision Neuroscience announced today that it received FDA 510(k) clearance for its Layer 7 cortical interface for BCI technology
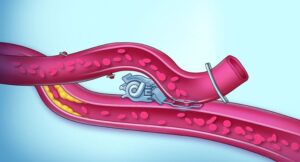
Adagio Medical (Nasdaq:ADGM) announced today that it received FDA breakthrough device designation for its vCLAS cryoablation system

SAN FRANCISCO, April 17, 2025 /PRNewswire/ — Solo Pace Incorporated, an emerging medical technology company, announced both FDA Clearance and First-In-Human use of their SoloPace Control System for temporary pacing in Transcatheter Aortic Valve Replacement (TAVR) procedures. With standardized workflows, the Control System is engineered to improve TAVR temporary pacing efficiency and reduce patient risks. Initial cases were completed this month at Scripps Clinic by Chief of Cardiology, Paul Teirstein, MD and Curtiss Stinis, MD.

DUBLIN, April 16, 2025 /PRNewswire/ — LUMA Vision Ltd., an innovative leader in advanced cardiac imaging and navigation, today announced the FDA clearance of its VERAFEYE™ Visualization Platform. This novel catheter-based imaging system provides real-time, two and four-dimensional, 360-degree visualization, significantly enhancing clinician precision and confidence during complex electrophysiology and structural heart procedures. The system is prepared for magnetic tracking and navigation of third-party catheters, which in the future, will further empower clinicians to perform complex procedures with greater precision and control.

PHILADELPHIA, April 15, 2025 /PRNewswire/ — ActiveProtective® (APT) is proud to announce the launch of Tango® Belt, a wearable medical device utilizing sensors to monitor motion and deploy airbags during serious hip-impacting falls. The Tango Belt, having already received Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA), was further granted marketing authorization as a prescription-only device intended as an adjunctive to standard-of-care to reduce the risk of hip fracture or hip dislocation due to falls in older adults at risk of major hip injury due to falls (DEN240021).
CeriBell (Nasdaq:CBLL) announced today that it received FDA 510(k) clearance for its next-generation Ceribell Clarity algorithm.
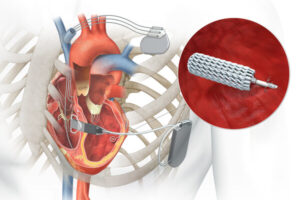
EBR Systems announced that it received FDA approval for its wireless cardiac pacing device for heart failure

DESKi today announced FDA clearance of HeartFocus, its AI-enabled heart exam software.