
Makani Science Gains FDA Clearance for Innovative Respiratory Monitoring Device – U.S.
Cutting-Edge Technology Enhances Real-Time Respiratory Tracking for Better Patient Care

Cutting-Edge Technology Enhances Real-Time Respiratory Tracking for Better Patient Care
Bright Uro announced today that it received FDA 510(k) clearance for its Glean urodynamic analyzer system.
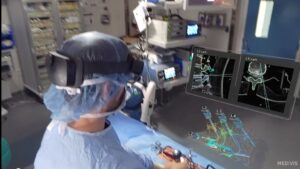
NEW YORK, April 2, 2025 /PRNewswire/ — Medivis Inc., a pioneer in surgical intelligence, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its groundbreaking Spine Navigation platform. This milestone marks a significant advancement in surgical technology, utilizing AR and AI to empower surgeons with holographic navigation across open and minimally invasive spine procedures. Alongside the FDA clearance, Medivis is announcing the commercial launch of Spine Navigation, making it available to hospitals and ambulatory surgical centers nationwide.

Teleflex (NYSE: TFX)+
announced today that it received FDA 510(k) clearance for its AC Range intra-aortic balloon pump (IABP).
Boston Scientific (NYSE: BSX) has received FDA clearance for its Bolt intravascular lithotripsy (IVL) system, according to the FDA’s 510(k) database.
PARIS, March 31, 2025 /PRNewswire/ — AZmed, one of the leading AI companies in medical imaging, today announced that it has received two new U.S. Food and Drug Administration (FDA) clearances for its AI tool AZchest. The clearances include applications intended to assist radiologists in the interpretation and detection of chest X-rays for lung nodules and triage capabilities for pneumothorax and pleural effusion.

SiBionics announced today that it unveiled its GS3 continuous glucose monitor (CGM), which now has CE mark approval.
SCOTTSDALE, Ariz., March 20, 2025 /PRNewswire/ — Anuncia Medical, Inc. (“Anuncia”), a pioneering company in CSF management and neurocritical care, has received Breakthrough Device Designation from the FDA for its ReFlow® EVD, an innovative solution for external ventricular drains (EVDs) used to manage brain swelling and elevated intracranial pressure.

Sibel Health announced today that it closed a $30 million equity financing and earned its seventh FDA 510(k) clearance.
Monogram Technologies secured 510(k) clearance for its robotic knee replacement system