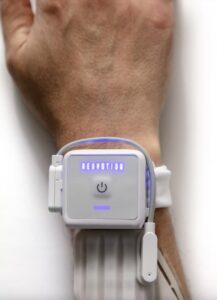
Aptitude Receives FDA Authorization for Metrix® COVID/Flu Multiplex Molecular Test for Point-of-Care and Over-the-Counter Use
SANTA BARBARA, Calif., Feb. 24, 2025 /PRNewswire/ — Aptitude Medical Systems, Inc. (Aptitude) today announced it has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its next-gen molecular Metrix® COVID/Flu multiplex test. This innovative test represents a major advancement in accessible molecular diagnostics.