
EndoQuest wins FDA IDE to study surgical robot in colorectal surgery
EndoQuest Robotics announced today that it received FDA investigational device exemption (IDE) for a study of its surgical robot.

EndoQuest Robotics announced today that it received FDA investigational device exemption (IDE) for a study of its surgical robot.
AngioDynamics today announced it received FDA 510(k) clearance for its NanoKnife system for prostate tissue ablation in patients with intermediate-risk prostate cancer.
Lungpacer Medical announced that it received FDA premarket approval for its flagship AeroPace neurostimulation system.
The solution offers clinical laboratories and healthcare providers a user-friendly, reliable, and cost-effective way to improve urine specimen collection, preservation, and transport.
LINKÖPING, Sweden, Dec. 6, 2024 /PRNewswire/ — SyntheticMR, leader in quantitative imaging software, is pleased to announce that its SyMRI 15 solution has obtained 510(k) clearance from the U.S. Food and Drug Administration (FDA) on diagnostic image replacement of conventional images.
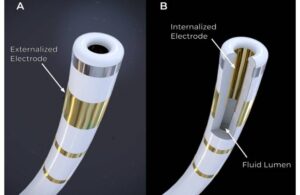
Field Medical announced today that it received FDA breakthrough device designation for its FieldForce pulsed field ablation (PFA) system.
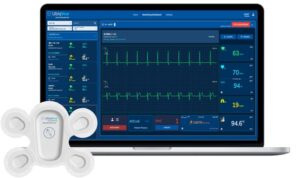
MILPITAS, Calif., Dec. 5, 2024 /PRNewswire/ — LifeSignals, Inc. today announced that the UbiqVue 2A Multiparameter System has received EU MDR Certification, marking another significant milestone in deploying continuous wireless patient monitoring for population health management following FDA 510(k) Clearance last month.
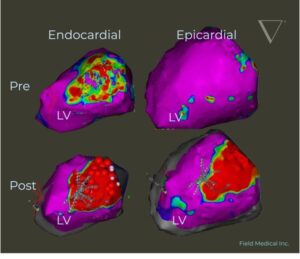
First and only contact force pulsed field ablation (PFA) system engineered to revolutionize care for the hundreds of thousands at risk of death from ventricular tachycardia (VT).
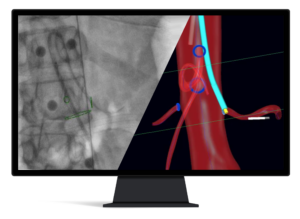
CLEVELAND, Dec. 4, 2024 /PRNewswire/ — Centerline Biomedical, Inc. (“Centerline”), an innovative leader in cardiovascular navigation and visualization systems, announced that the company has received US Food and Drug Administration (FDA) 510(k) clearance for its new IOPS Guidewire Handle

ShiraTronics announced that it initiated its FDA investigational device exemption (IDE) pivotal trial for its neuromodulation therapy.