Magstim earns FDA clearance for transcranial magnetic stimulation that treats depression, OCD
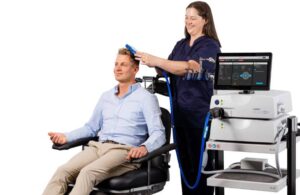
Magstim announced today that the FDA cleared its next-generation Horizon Inspire system for delivering transcranial magnetic stimulation (TMS).

Magstim announced today that the FDA cleared its next-generation Horizon Inspire system for delivering transcranial magnetic stimulation (TMS).
iCad (Nasdaq:ICAD) announced today that the FDA cleared its ProFound Detection version 4.0 for digital breast tomosynthesis (DBT).
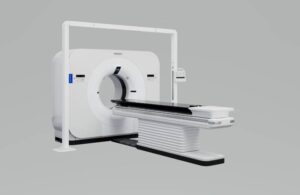
Philips (NYSE:PHG) announced today that the FDA granted 510(k) clearance for its new detector-based spectral computed tomography (CT) radiotherapy solution.

TEL AVIV, Israel and FORT LAUDERDALE, Fla., Nov. 11, 2024 /PRNewswire/ — Momentis Surgical Ltd., a leader in robotic-assisted surgical innovation, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for its second-generation Anovo® robotic surgical platform.

IRVINE, Calif., Nov. 7, 2024 /PRNewswire/ — Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, today announced the U.S. Food & Drug Administration (FDA) approval of the VARIPULSE™ Platform for the treatment of drug refractory paroxysmal Atrial Fibrillation (AFib).
Corin announced that it received FDA 510(k) clearance for its Icona femoral stem implant for total hip arthroplasty (THA).

BOSTON, Nov. 6, 2024 /PRNewswire/ — Zeta Surgical announced today that its Zeta Navigation System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with expanded instruments and enhanced hospital connectivity.
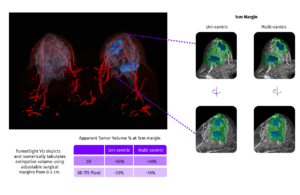
SimBioSys announced that it received its second FDA 510(k) clearance for TumorSight Viz to expand its use by breast surgeons in the U.S.

Akura Medical, a Shifamed portfolio company, announced today that the FDA granted its Katana system investigational device exemption (IDE).

NeurAxis (NYSE:NRXS) announced today that the FDA granted an expanded 510(k) clearance for its IB-Stim non-implanted nerve stimulator.