
Cordis wins FDA nod for vascular closure device
Cordis announced today that the FDA granted premarket approval (PMA) for its Mynx Control venous vascular closure device (VCD).

Cordis announced today that the FDA granted premarket approval (PMA) for its Mynx Control venous vascular closure device (VCD).

Pulse Biosciences (Nasdaq:PLSE) announced today that the FDA granted breakthrough device designation for its pulsed field ablation system. Shares of PLSE rose more than 20% in mid-morning trading on the back of this announcement.
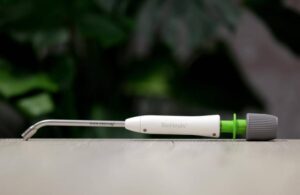
Signum Surgical announced today that the FDA granted de novo clearance for its BioHealx implant for treating anal fistula.

IceCure Medical (Nasdaq:ICCM) announced today that the FDA granted clearance for its next-generation single-probe cryoablation system

Each patch now replaces up to 12 mealtime injections, equaling more than 1,000 fewer injections annually, according to a news release.

SILVER SPRING, Md., June 27, 2024 /PRNewswire/ — Today, the U.S. Food and Drug Administration granted marketing authorization to Cepheid for the Xpert HCV test and GeneXpert Xpress System, the first hepatitis C virus (HCV) test that can be used to bring diagnosis to appropriately certified point-of-care settings for individuals at risk for hepatitis C.

WASHINGTON, June 26, 2024 /PRNewswire/ — MCRA, the leading privately held independent medical device, diagnostics, and biologics Clinical Research Organization (CRO) and advisory firm is pleased to announce its role in aiding CamDiab’s artificial pancreas software, CamAPS FX, in achieving U.S. Food and Drug Administration (FDA) clearance.

Front Line Medical Technologies has announced that its COBRA-OS (Control Of Bleeding, Resuscitation, Arterial Occlusion System) has officially been granted CE marking under the new European Medical Device Regulations.
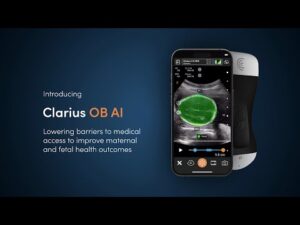
The company’s 8th AI model makes ultrasound easier for new ultrasound users to capture important fetal measurements

Powered by this AI, the Pocket-Sized Kardia 12L ECG System Enables Faster, Easier Detection of Life-Threatening Cardiac Conditions