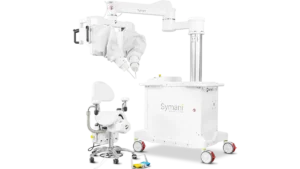
MMI receives de novo nod for microsurgery robot
Medical Microinstruments said the system could increase the number of physicians who can perform complicated microsurgical procedures.

Medical Microinstruments said the system could increase the number of physicians who can perform complicated microsurgical procedures.

Neurovalens announced today that it received FDA clearance for its Modius Stress device for treating anxiety and raised $2.65 million.
Orthobond has secured FDA de novo approval for its Ostaguard antibacterial technology that could one day be used for a wide range of medical devices — and beyond medtech.

Siemens Healthineers announced today that it received FDA 510(k) clearance for its MammoMat B.brilliant mammography platform.
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
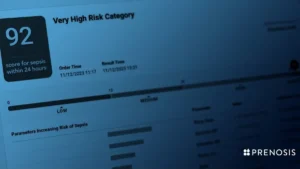
The CEO of Prenosis told MedTech Dive the company sees third-party validation as important, with the FDA having clarified that certain decision support tools should be regulated as medical devices.

TEG testing provides critical information that can help physicians improve hemostasis management for their patients

HeadaTerm 2 uses neuromodulation technology. It releases targeted electrical impulses that increase the pain tolerance of the wearer.
The Venus Versa Pro combines the applicator of the Venus Viva MD with the Venus Versa system which are both approved in Australia and registered in Australian Register of Therapeutic Goods (ARTG).

This milestone gives healthcare professionals an important tool for managing bacteremia by providing antibiotic susceptibility test (AST) results with unprecedented speed.