
Tyber Medical Receives FDA Clearance on Plating System Line Extension
Tyber Medical Broadens Its Vast Plating Portfolio with FDA Approval of Additional Screw and Plate Options, and Indications for Mini-Frag System

Tyber Medical Broadens Its Vast Plating Portfolio with FDA Approval of Additional Screw and Plate Options, and Indications for Mini-Frag System

The device is designed to reduce the risk of hernia by distributing suture tension over a large area of tissue.

Element Science announced that it received CE mark approval for its novel patch-wearable cardioverter defibrillator (P-WCD).

The single-use, PEEK-based RIB System utilizes the platform technology of Able’s Valkyrie® Thoracic Fixation System, further strengthening Able Medical’s portfolio.
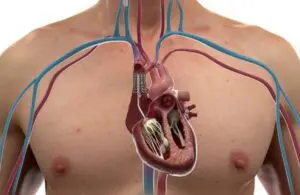
CroíValve announced today that it began an early feasibility study for its Duo tricuspid coaptation valve system.

Abbott (NYSE: ABT)+
announced today that it received approval from the FDA for the launch of its Liberta RC DBS system
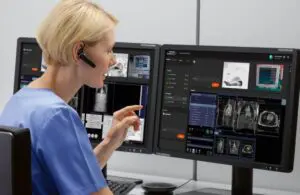
Siemens Healthineers announced that the FDA cleared its Syngo Virtual Cockpit, a private, secure communication platform.

Intuitive Surgical (Nasdaq: ISRG)+
announced today that it received CE mark approval for its da Vinci SP surgical robot platform.

SnoreLessNow announced today that the FDA granted 510(k) clearance for its over-the-counter dental device.
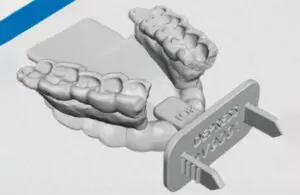
Kallisio recently announced that the FDA has cleared its 3D-printed Stentra, used to protect head and neck cancer patient’s healthy tissue during radiation therapy.