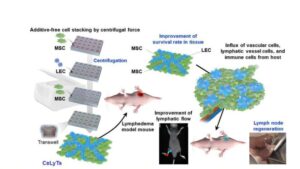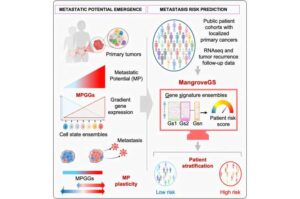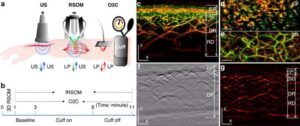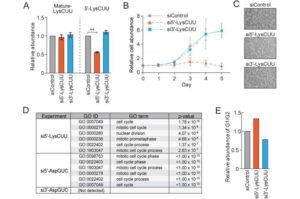
Tiny molecules called tRNA halves may contribute to prostate cancer cell growth
Prostate cancer is the second-most common cancer in men. A new study from Thomas Jefferson University uncovered a new potential therapeutic target in tiny molecules called tRNA halves.