FDA clears Cepheid’s Xpert GI Panel for pathogen detection
The Xpert GI Panel identifies pathogens directly from stool specimens collected in Cary-Blair transport media.

The Xpert GI Panel identifies pathogens directly from stool specimens collected in Cary-Blair transport media.
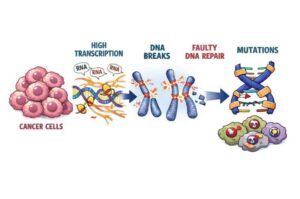
A new study shows that cancer damages its own DNA by pushing key genes to work too hard. Researchers found that the most powerful genetic “on switches” in cancer cells, called super-enhancers, drive unusually intense gene activity. That high gear creates stress on the DNA and can cause dangerous breaks.
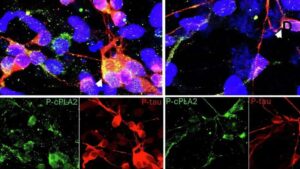
A multidisciplinary team has developed a selective compound that inhibits an enzyme tied to inflammation in people at genetic risk for Alzheimer’s, while preserving normal brain function and crossing the blood-brain barrier.
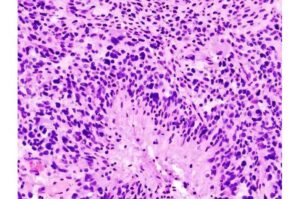
A team of Canadian scientists has uncovered a new way to slow the growth of glioblastoma, the most aggressive and currently incurable form of brain cancer—and identified an existing medication that could treat it.

Researchers have identified a metabolically sensitive cell subtype in the eye’s drainage system which shows early signs of dysfunction in a genetic mouse model of glaucoma.
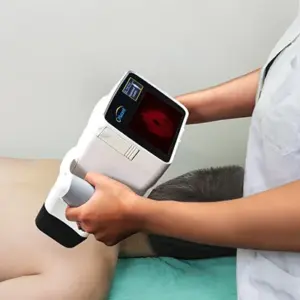
LOS GATOS, Calif., Jan. 20, 2026 /PRNewswire/ — In the pivotal clinical trial of their technology, researchers at Orlucent®, Inc. have confirmed the accuracy of the company’s Skin Fluorescent Imaging (SFI) System, a non-invasive, hand-held molecular imaging device for the direct, on-the-skin, point-of-care assessment of suspicious moles and early detection of melanoma-related activity.

FDA-cleared PCR test aids in detection of 11 diarrhea-causing bacteria, viruses, and parasites from 1 sample

The FDA-approved comprehensive genomic profiling test will be reimbursed at a rate of $2,989.55 per test, helping to advance adoption in the US healthcare system
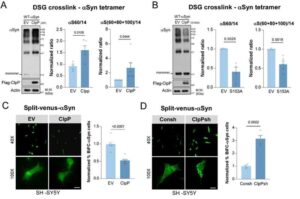
About 1 million Americans suffer from Parkinson’s disease, with around 90,000 new cases diagnosed each year, according to the Parkinson’s Foundation. The chronic, degenerative brain disorder destroys dopamine-producing cells essential for smooth, coordinated movement.
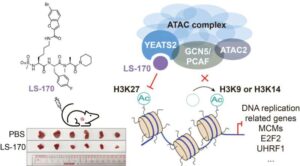
A research team has made a breakthrough in epigenetic drug discovery. The researchers have successfully developed a first-in-class chemical inhibitor that precisely and selectively targets the ATAC complex, a critical cellular “switch operator” that activates tumor-promoting genes, opening a novel therapeutic avenue for non-small cell lung cancer (NSCLC).