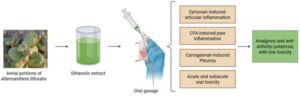
Plant used in folk medicine has anti-inflammatory anti-arthritic effects, study confirms
In Brazil, researchers from the Federal University of Grande Dourados (UFGD), the State University of Campinas (UNICAMP), and São Paulo State University (UNESP) have conducted a study that confirmed the safety and anti-inflammatory, analgesic, and anti-arthritic properties of the Joseph’s Coat plant (Alternanthera littoralis).