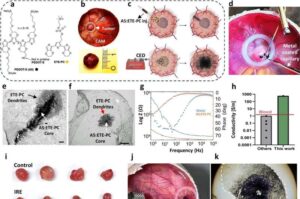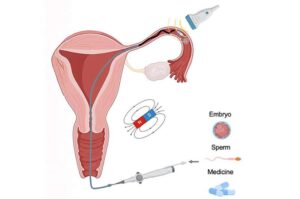
Magnetic microcatheters offer precise, minimally invasive delivery for reproductive medicine
A new international study led by the Nanobiosystems group at CIC nanoGUNE, is developing miniature, non-invasive, precise robotic catheters for use in reproductive medicine and gynecological health.