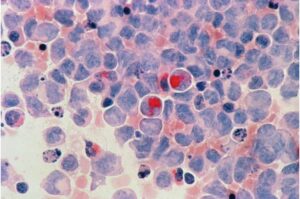
Hidden nuclear droplets link multiple leukemias and reveal a new therapeutic target
A hidden structure inside the cell is rewriting how scientists understand leukemia. Beneath the microscope, what looked like disorder turned out to follow a simple physical rule—one that connects several major mutations behind the disease.