
Onkos Surgical wins FDA de novo nod for antibacterial-coated orthopedic implants
Onkos Surgical this week announced it received FDA de novo approval for its antibacterial coated implants.

Onkos Surgical this week announced it received FDA de novo approval for its antibacterial coated implants.

Ross Bjella, Kelyniam’s CEO, said, “This approval will further boost Kelyniam’s sales momentum which started in Q4 last year

SurGenTec announced today that it received FDA 510(k) clearance for its OsteoFlo HydroPutty synthetic bone graft.

Medtronic (NYSE: MDT)+
announced today that it received FDA 510(k) clearance for its OsteoCool 2.0 bone tumor ablation system.

Nevro1™ Proven to Immediately Transfix Sacroiliac (SI) Joint to Allow for Long-term SI Joint Fusion

BETHLEHEM, Pa., Feb. 6, 2024 /PRNewswire/ — Tyber Medical LLC, a leading orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, received clearance for the anatomical plating system in Canada. The comprehensive portfolio previously received FDA 510(k) in the US and has now been cleared through Health Canada.

Tyber Medical Broadens Its Vast Plating Portfolio with FDA Approval of Additional Screw and Plate Options, and Indications for Mini-Frag System
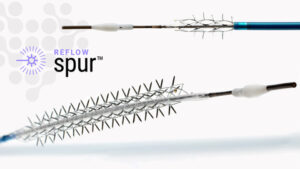
Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System.
When injected, Renovite acts as a scaffold for cells and helps localize and retain molecules that stimulate healing
DePuy Synthes, the orthopedic device business of Johnson & Johnson (NYSE: JNJ)+
, has won FDA 510(k) clearance for its TriLeap lower extremity anatomic plating system.