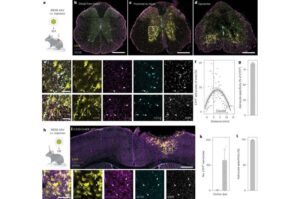
How the nervous system activates repair after spinal cord injury
After a spinal cord injury, cells in the brain and spinal cord change to cope with stress and repair tissue. A new study from Karolinska Institutet, published in Nature Neuroscience, shows that this response is controlled by specific DNA sequences. This knowledge could help develop more targeted treatments.