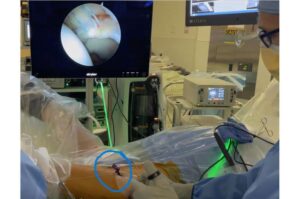
icotec Receives FDA Clearance for the CMORE® CT System, Expanding its BlackArmor® Engineered Carbon/PEEK Technology to the Posterior Cervicothoracic Spine
ALTSTÄTTEN, Switzerland and BOSTON, Nov. 13, 2025 /PRNewswire/ — icotec, the pioneer of implantable devices made from BlackArmor® Engineered Carbon/PEEK, today announced that the U.S. Food and Drug Administration (FDA) has cleared the CMORE® CT System for use in the cervicothoracic spine.