
Stereotaxis surgical robot earns regulatory nod in China
Stereotaxis (NYSE:STXS) announced today that its Genesis surgical robot received regulatory approval in China.

Stereotaxis (NYSE:STXS) announced today that its Genesis surgical robot received regulatory approval in China.
Globus Medical (NYSE:GMED) announced that launched its ExcelsiusHub navigation system for robotic surgical procedures.

FREMONT, Calif., Nov. 18, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with the LinkSymphoKnee (LSK) from Waldemar Link GmbH & Co. KG, Germany (LINK) under a Collaboration Agreement between the two companies.
Johnson & Johnson MedTech announced today that the FDA granted its Ottava surgical robot investigational device exemption (IDE).

TEL AVIV, Israel and FORT LAUDERDALE, Fla., Nov. 11, 2024 /PRNewswire/ — Momentis Surgical Ltd., a leader in robotic-assisted surgical innovation, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for its second-generation Anovo® robotic surgical platform.
Corin announced that it received FDA 510(k) clearance for its Icona femoral stem implant for total hip arthroplasty (THA).

Distalmotion announced today that it received FDA de novo approval for its Dexter surgical robot for adult inguinal hernia repair.
MOUNTAIN VIEW, Calif., Oct. 24, 2024 /PRNewswire/ — In a groundbreaking advancement for medical science, surgeons in Santiago, Chile, performed the world’s first robotic surgeries combining 3D visualization and augmented reality (AR), marking a significant step forward in improving surgical speed, visualization, precision, and patient outcomes.
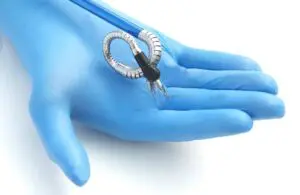
Momentis Surgical (formerly Memic) announced today that it received FDA 510(k) clearance for its Anovo robotic surgical platform.
CMR Surgical announced today that it received FDA de novo clearance to market its Versius surgical robot platform.