
Medtronic’s blood pressure device wins coverage nod in Japan
Medtronic is investing in the rollout of the Symplicity Spyral renal denervation procedure, including hiring for new market development roles, CEO Geoff Martha said on an earnings call.

Medtronic is investing in the rollout of the Symplicity Spyral renal denervation procedure, including hiring for new market development roles, CEO Geoff Martha said on an earnings call.

The company has integrated its Eversense 365 implant with Sequel Med Tech’s insulin delivery system to automate the management of Type 1 diabetes.
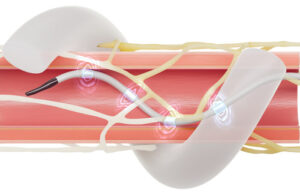
Medtronic continues to expand the growth opportunities for its hypertension-treating Symplicity Spyral renal denervation system.
GE HealthCare (Nasdaq: GEHC)+ announced today that it received FDA clearance for a trio of new MRI offerings.

LOUISVILLE, Ky., Feb. 19, 2026 /PRNewswire/ — Breath Diagnostics, Inc., a leader in breath-based molecular diagnostics powered by patented microreactor capture technology, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to its OneBreath™ platform.

DAYTON, Ohio, Feb. 19, 2026 /PRNewswire/ — Innovative Sterilization Technologies (IST) announced today that the ONE TRAY® sterilization container has received a second clearance from the U.S. Food and Drug Administration (FDA), significantly expanding its use across hospital and surgical facility workflows.

CHICAGO, Feb. 19, 2026 /PRNewswire/ — Sibel Health, a leader in medical-grade wearable sensor technology, today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s recent Letter of Intent (LOI) into the Clinical Outcome Assessment (COA) Qualification Program under the Drug Development Tool (DDT) framework. The acceptance marks a significant milestone in advancing objective cough frequency measurement for adult patients with chronic refractory cough (CRC) using a novel Cough Monitoring sensor, the Aria sensor.
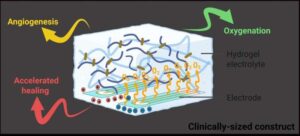
As aging populations and rising diabetes rates drive an increase in chronic wounds, more patients face the risk of amputations. UC Riverside researchers have developed an oxygen-delivering gel capable of healing injuries that might otherwise progress to limb lo
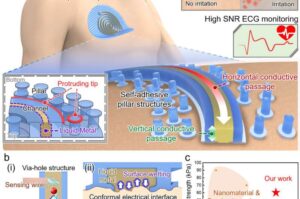
Conventional ECG patches often require cold gels and adhesives, which can cause skin irritation and leave marks. These materials can also lose effectiveness during vigorous movement, compromising signal quality. Addressing these issues, UNIST researchers have developed a novel, self-adhesive ECG patch that eliminates the need for gels and chemical adhesives.

Researchers at the Icahn School of Medicine at Mount Sinai have reported promising findings that may help redefine treatment for patients with muscle-invasive bladder cancer, a potentially aggressive form of the disease traditionally treated with surgical removal of the bladder. The study, published in the Proceedings of the National Academy of Sciences, demonstrates that ultra-sensitive testing of tumor-derived DNA in blood and urine may help identify patients who can safely preserve their bladder without compromising cancer outcomes.