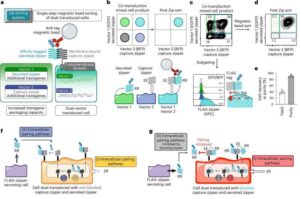
Smartwatch app uses motion sensors to help smokers quit
Whether people decide to make New Year’s resolutions or not, they might want to lead a healthier lifestyle in 2025. According to a new University of Bristol-led study, smartwatches could help people give up smoking.