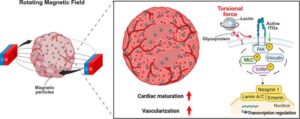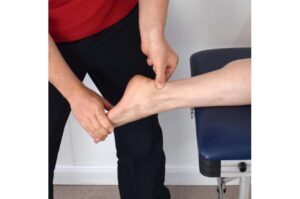
HIF1 protein identified as key trigger in common tendon diseases
Complaints such as pain in the Achilles tendon, tennis elbow, swimmer’s shoulder and jumper’s knee are familiar to many young sportspeople, as well as to older individuals. These conditions are all caused by overloading of tendons and are generally very painful.