Handheld device offers lab-quality diagnostic testing
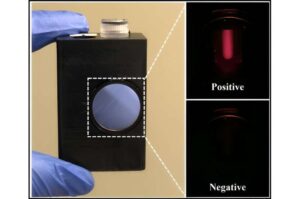
Because of its high accuracy, laboratory-based polymerase chain reaction (PCR) testing is the gold standard for infectious disease diagnostics. However, PCR technology requires highly trained staff and costly equipment, hindering its availability, especially in low-resource settings. New research suggests a different kind of test could be more streamlined without sacrificing performance