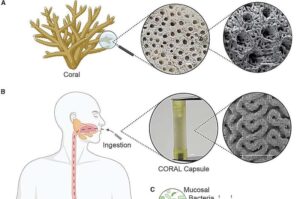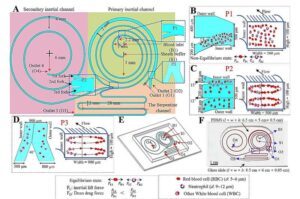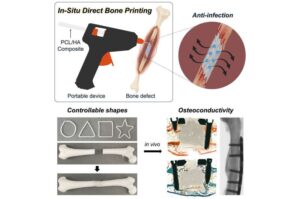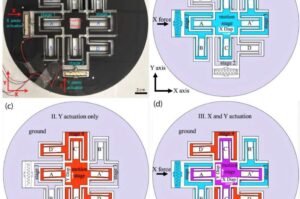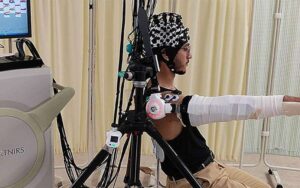
Cyborg-type robots can boost neuroplasticity when users control their own movement
Researchers at University of Tsukuba have demonstrated for the first time that brain regions responsible for high-level motor planning and preparation, such as the premotor cortex, are activated when a cyborg-type robot is volitionally controlled.