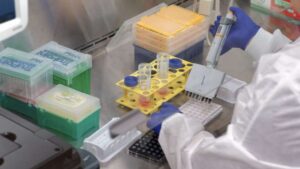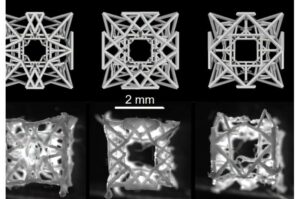
3D-printed cardiac patch encourages tissue repair using structural mesh and hydrogel with living cells
A new type of tissue-engineered cardiac patch could not only seal defective areas of the heart, as has been the case up to now, but also heal them. An interdisciplinary team led by ETH Zurich has successfully implanted the patch in animals.