
Olympus picks up pediatric indications from FDA for duo of single-use bronchoscopes
Olympus announced today that it received FDA clearance for pediatric use for two of the single-use bronchoscopes it distributes in the U.S.

Olympus announced today that it received FDA clearance for pediatric use for two of the single-use bronchoscopes it distributes in the U.S.

BOSTON, Feb. 23, 2026 /PRNewswire/ — Haemonetics Corporation (NYSE: HAE), a global medical technology company focused on delivering innovative solutions designed to improve patient outcomes, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the NexSys PCS® Plasma Collection System with Persona® PLUS technology. Persona PLUS represents the next generation of Haemonetics’ proprietary and patented Persona technology that tailors plasma collections to each donor for improved average plasma volume per donation.
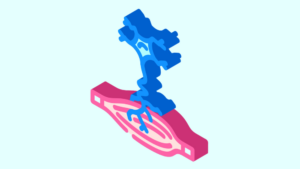
ATLANTA, Feb. 23, 2026 /PRNewswire/ — MiRus® today announced FDA 510(k) clearance and commercial launch of the IO™ Expandable Wedge Osteotomy System, an innovative solution designed to bring intraoperative precision and adjustability to foot and ankle osteotomy procedures.
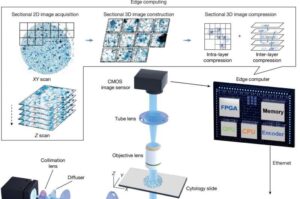
One way cancer specialists detect the disease is by examining cells and bodily fluids under a microscope, a time-consuming and labor-intensive process called cytology. It involves visually inspecting tens of thousands to one million cells per slide for subtle 3D morphological changes that might signal the onset of cancer. But AI offers an approach that is potentially faster and more accurate.
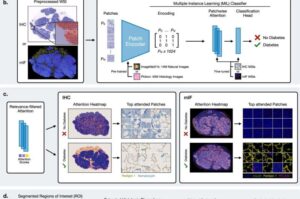
Researchers from several partner institutions of the German Center for Diabetes Research (DZD) have collaborated with international colleagues to develop a new approach for visualizing subtle tissue changes in the pancreas in type 2 diabetes. The results provide new insights into the development of type 2 diabetes. The study has now been published in Nature Communications.

Using machine learning, an electronic nose can “smell” early signs of ovarian cancer in the blood. The method is precise and, according to the LiU researchers behind the study, it could eventually be used to find many different cancers. The study is published in Advanced Intelligent Systems.

New research from UCLA Health suggests that certain inhalers used to treat chronic obstructive pulmonary disease (COPD) are not only less harmful to the environment but can also lead to slightly better patient outcomes. Inhalers are essential therapies for COPD and other lung conditions, but many commonly used devices rely on propellants that are potent greenhouse gases.
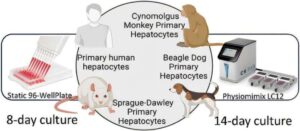
Creating a drug that might help treat or cure a health condition in humans is a long, complex process. After developing a candidate drug that shows potential—a process that, in and of itself, can take decades—scientists often spend years testing the safety of new medications in cells, animals and then in humans.
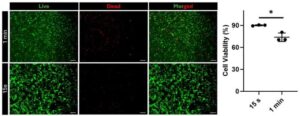
A multidisciplinary team have built hydrogels built entirely from synthetic peptides so their properties can be precisely tailored through chemical design. By harnessing the power of collagen-inspired peptides and light-triggered chemistry, a University of Ottawa research team has engineered a customizable material with the potential to be a gamechanger for soft tissue repair, whether it’s closing a surgical incision or sealing a traumatic wound.

The key defining features of Thermobalancing therapy are its high treatment efficacy and no side effects