MedTech News
.................... by Andrew Celentano

Onkos Surgical® Receives FDA 510(k) Clearance for ELEOS™ Proximal Tibia with NanoCept® Antibacterial Technology
PARSIPPANY, N.J., Oct. 21, 2025 /PRNewswire/ — Onkos Surgical, a leading provider of innovative solutions for complex orthopaedic procedures, announced that it has recently received a 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ELEOS Proximal Tibia with NanoCept Antibacterial Technology. This marks the first 510(k) clearance for the NanoCept Antibacterial Technology since the original De Novo authorization was granted in April of 2024.
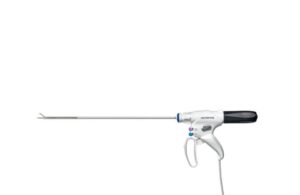
Olympus launches new surgical energy device for hemostatic cutting, vessel sealing
Olympus announced today that it launched Thunderbeat II, its next-generation hybrid energy device for soft tissue management.

Aesculap Launches Irrigating Disposable Bipolar Forceps with Non-Stick Technology
CENTER VALLEY, Pa., Oct. 20, 2025 /PRNewswire/ — Aesculap, Inc., a leader in surgical innovation, announced the launch of its new Irrigating Specialty Non-Stick Disposable Bipolar Forceps to the U.S. market. Designed with surgical excellence in mind, the forceps are engineered to enhance precision, efficiency and intuitive handling in the operating room.
AI system delivers comprehensive cancer diagnosis
The Hong Kong University of Science and Technology (HKUST) today launched SmartPath, a comprehensive artificial intelligence (AI) system designed to transform the entire pathology workflow for cancer care.
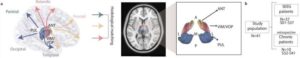
Personalized brain stimulation offers new hope for people with hard-to-treat epilepsy
Doctors and researchers at the University of Pittsburgh and UPMC have developed a new treatment for epilepsy patients who don’t respond to medication and aren’t candidates for surgery. Their approach, published in Nature Communications, uses deep brain stimulation (DBS) that is tailored to each patient’s unique brain wiring.
Electronic eye implant and AR glasses restore reading vision in people with sight loss
After being treated with an electronic eye implant paired with augmented-reality glasses, people with sight loss have recovered reading vision, reports a trial involving a UCL (University College London) and Moorfields Eye Hospital clinical researcher.

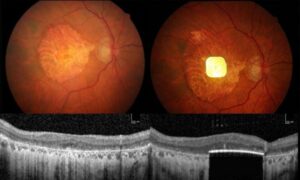
Retinal implant restores central vision in patients with advanced AMD, study shows
A wireless retinal implant can restore central vision in patients with advanced age-related macular degeneration (AMD), according to clinical trial results published in the New England Journal of Medicine.
Rectal “breathing” possible emergency treatment
The technique sounds so outlandish that it won an IgNobel prize in 2024. But the science behind rescuing people with blocked airways and clogged lungs by rectally delivering oxygen to the body is no joke.
