MedTech News
.................... by Andrew Celentano

Could Fat-Trapping Boba Pearls Replace Weight Loss Medications Like GLP-1 Agonists?
Learn how microbeads made from green tea polyphenols, vitamin E, and seaweed could help break down fats without the negative side effects that can come from GLP-1 agonists.

Tosoh Bioscience Receives FDA 510(k) Clearance for Fast, Accurate GR01 HbA1c Testing Analyzer
GROVE CITY, Ohio, Oct. 30, 2025 /PRNewswire/ — Tosoh Bioscience, Inc., a market leader in clinical diagnostics has received U.S. FDA 510(k) clearance for its next-generation Tosoh Automated Glycohemoglobin Analyzer HLC®-723 GR01 (GR01) for HbA1c testing.
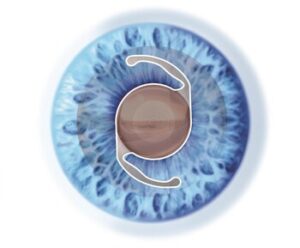
Revolutionary Cataract Surgery Technology Transforms Eye Care with Precision and Enhanced Patient Outcomes
SCOTTSDALE, Ariz., Oct. 30, 2025 /PRNewswire/ — In an exciting breakthrough for eye health, Barnet Dulaney Perkins, a leader in ophthalmic innovation, uses an advanced cataract surgery technique that delivers faster recovery, increased precision, and exceptional long-term results. The approach, which incorporates cutting-edge technology, revolutionizes how cataracts are treated, benefiting millions of patients worldwide.

Laser eye treatment shows potential for halting dry macular degeneration progression in animal models
Around a third of people over the age of 80 suffer from age-related macular degeneration (AMD), with an estimated 20 million Americans aged 40 and older currently living with AMD.

An app, an Apple Watch and AI: A new way for researchers to study sleep health
An app that turns consumer Apple Watches into tools for highly sophisticated sleep stage monitoring was developed by a team of researchers led by professor Joyita Dutta at the University of Massachusetts Amherst

Betterguards launches The BetterGuard Lite Ankle Protection System
The BetterGuard Lite features an impossibly thin, lightweight design at an affordable price for athletes worldwide

Tandem launches t:slim X2 pump with Abbott FreeStyle Libre 3 Plus
Tandem Diabetes Care (Nasdaq:TNDM) announced today that it launched its t:slim X2 insulin pump with the Abbott (NYSE:ABT) FreeStyle Libre 3 Plus CGM.
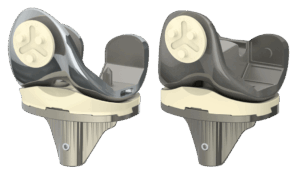
Patients Enjoying Faster Recoveries thanks to Optimotion Implants
ORLANDO, Fla., Oct. 29, 2025 /PRNewswire/ — Optimotion Implants, a medical device company that designs and manufactures total knee replacement implants and associated surgical instrumentation is expanding operations in the Tampa Bay area. Optimotion Implants is the only company in the world utilizing the revolutionary Lateral Approach technique in total knee replacement. Participating surgeons are highly trained on the Lateral Approach which allows them to do what others cannot, leading to better outcomes for patients.
