MedTech News
.................... by Andrew Celentano
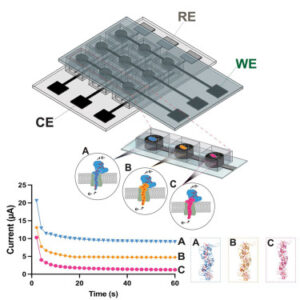
Bioelectrochemical crossbar architecture screening platform for extracellular electron transfer
Bioelectronic devices link microbes and materials to convert chemical signals into electrical outputs for sensing, energy, and synthesis. Unlocking this potential requires systems that can interrogate electroactive cells rapidly and at scale, but traditional bioelectrochemical platforms remain costly, bulky, and low throughput.
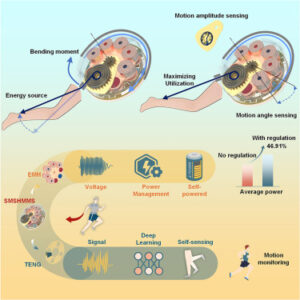
Self-aligning mechanism for wearable biomechanical energy harvesters
The increasing demand for real-time, wireless, and sustainable motion monitoring in applications such as personal health, professional sports, and human-machine interactions calls for wearable devices that are lightweight and battery free.

QuidelOrtho Receives FDA 510(k) Clearance for VITROS™ Immunodiagnostic Products hs Troponin I Assay
SAN DIEGO, Nov. 3, 2025 /PRNewswire/ — The U.S. Food and Drug Administration (“FDA”) has granted QuidelOrtho Corporation (Nasdaq: QDEL) (“QuidelOrtho”), a global leader of in vitro diagnostics, 510(k) clearance for the VITROS hs Troponin I Reagent Pack (the “VITROS hs Troponin I Assay”). The assay is intended for the quantitative measurement of cardiac troponin I (cTnI) in human plasma (heparin) to aid in the diagnosis of myocardial infarction (MI).

EmeTerm Wristband Significantly Reduces Postoperative Nausea and Vomiting in Major Orthopedic Surgeries
VANCOUVER, BC, Nov. 3, 2025 /PRNewswire/ — WAT Medical Enterprise Ltd. proudly announced the publication of a groundbreaking clinical trial in The Journal of Bone & Joint Surgery (JBJS) which demonstrates the significant benefits of the EmeTerm wristband in reducing postoperative nausea and vomiting (PONV) in patients undergoing total hip or knee arthroplasty (THA/TKA) under spinal anesthesia.

Biosensor technology may lead to breath test for lung cancer
University of Texas at Dallas researchers have developed biosensor technology that, when combined with artificial intelligence (AI), shows promise for detecting lung cancer through breath analysis.
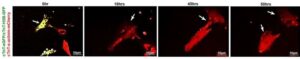
Specific human gene can help the heart repair itself from heart attack or heart failure
A naturally occurring gene called Cyclin A2 (CCNA2), which turns off after birth in humans, can actually make new, functioning heart cells and help the heart repair itself from injury, including a heart attack or heart failure, when the gene is turned back on.

Neural implant smaller than a grain of salt can wirelessly track brain
Cornell University researchers and collaborators have developed a neural implant so small that it can rest on a grain of salt, yet it can wirelessly transmit brain activity data in a living animal for more than a year.
Turning on an immune pathway in tumors could lead to their destruction
MIT researchers show they can use messenger RNA to activate the pathway and trigger the immune system to attack tumors.
