MedTech News
.................... by Andrew Celentano
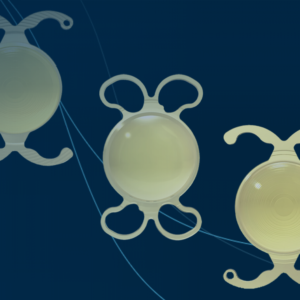
BVI wins FDA nod for trifocal intraocular lens
BVI announced today that the FDA approved its FineVision HP trifocal intraocular lens (IOL) for cataract surgery.

Zeta Surgical wins FDA clearance for TMS navigation system
Zeta Surgical this week announced it received FDA 510(k) clearance for its Zeta TMS Navigation System
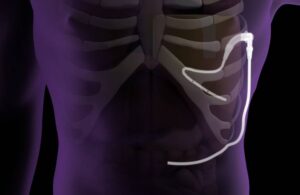
Pleural Dynamics earns Medicare win for effusion shunt
Pleural Dynamics announced today that it received a new CMS code for its ACES (automatic continuous effusion shunt) device.
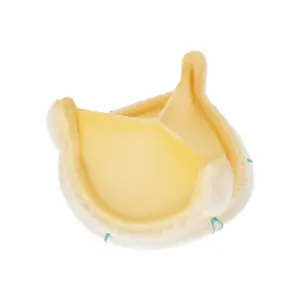
Medtronic launches Avalus Ultra surgical valves in India
Avalus Ultra valve expands Medtronic Cardiac Surgery’s portfolio of cardiac surgical innovations

FDA clears Roche’s blood-based test for Alzheimer’s assessment
The blood-based biomarker test was developed in partnership with Eli Lilly and Company.

MSU develops new wearable device to support trial participant safety
MSU’s Technology Transfer Office is facilitating the commercialisation process of the device.

ESMO 2025: Lunit’s AI tool predicts immunotherapy response across cancer types
Three presentations at ESMO 2025 will demonstrate Lunit SCOPE IO’s value in predicting immunotherapy response in colorectal, kidney, and lung cancer patients.
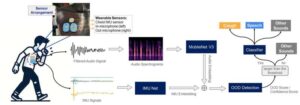
Improved cough-detection tech can help with health monitoring
Researchers have improved the ability of wearable health devices to accurately detect when a patient is coughing, making it easier to monitor chronic health conditions and predict health risks such as asthma attacks. The advance is significant because cough-detection technologies have historically struggled to distinguish the sound of coughing from the sound of speech and nonverbal human noises.
