MedTech News
.................... by Andrew Celentano
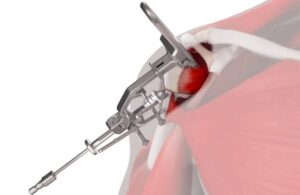
Johnson & Johnson MedTech launches Inhance Intact shoulder instrumentation
ohnson & Johnson MedTech (NYSE: JNJ)+
today announced the launch of its Inhance Intact shoulder arthroplasty instrumentation system.

Medtronic earns first-of-its-kind FDA labeling for Endurant stent graft
Medtronic (NYSE: MDT)+
announced today that it received new labeling approval from the FDA for its Endurant stent graft system.
AI models predict sepsis in children
Health data helped identify kids in the ER who are likely to develop sepsis within 48 hours
FDA grants 510(k) clearance to Surgical Theater’s spine platform
OrthoIndy spine surgeon Dr Greg Poulter performed the inaugural SyncAR Spine case.
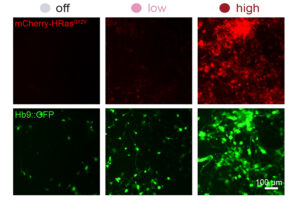
A new system can dial expression of synthetic genes up or down
The promoter editing system could be used to fine-tune gene therapy or to more efficiently reprogram cells for therapeutic use.
Smartee Launches World’s First Sleep Aligner Combining Anti-Snoring Therapy with Teeth Alignment
SHANGHAI, Oct. 11, 2025 /PRNewswire/ — Smartee Denti-Technology today announced the global launch of the Smartee Sleep Aligners, a clear aligner device designed to help manage obstructive sleep apnoea hypopnoea syndrome (OSAHS) and primary snoring (PS). The new series includes two versions: Smartee SA, which focuses on the treatment of OSAHS and PS, and Smartee SA Plus. The latter can treat OSAHS while simultaneously addressing orthodontic issues. Together, they provide clinicians with an invisible alternative to conventional OSAHS treatment therapies, offering greater comfort and compliance, and improving sleep quality.
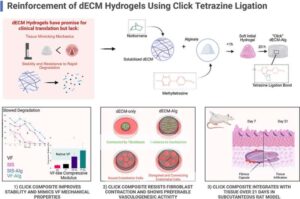
New injectable gel shows promise as voice loss treatment
McGill University researchers have engineered a new hydrogel that shows early promise as a treatment for people with vocal cord injuries.

How Eggs Can Harbor Salmonella, Even When They Look Perfectly Clean
How do eggs get Salmonella? This bacteria poses a real threat to many eggs, but can easily be killed by cooking at a high heat.
