MedTech News
.................... by Andrew Celentano

Avalanches: User-carried safety device can increase survival time fivefold
When the Norwegian company that manufactures the Safeback SBX device, which is already on the market, approached Eurac Research to have it independently tested, it was clear that the international research team led by physician and researcher Giacomo Strapazzon would publish the results of the study in any case, regardless of the outcome.

Scientists are collecting toenail clippings to reveal radon exposure and lung cancer risk
At 47 years of age, Emi Bossio was feeling good about where she was. She had a successful law practice, two growing children and good health. Then she developed a nagging cough. The diagnosis to come would take her breath away.
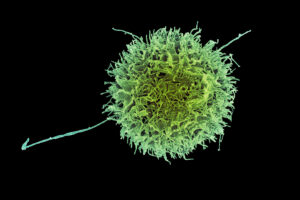
Engineered “natural killer” cells could help fight cancer
A new study identifies genetic modifications that make these immune cells, known as CAR-NK cells, more effective at destroying cancer cells.

Armor Medical wins MedTech Innovator 2025 Grand Prize at The MedTech Conference
MedTech Innovator held its Grand Prize Finals at AdvaMed’s the MedTech Conference, with Armor Medical coming away the winner.
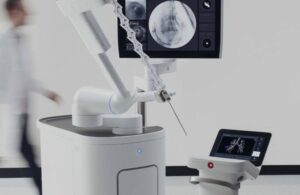
Intuitive gets expanded FDA nod for AI, imaging integration for Ion
ntuitive Surgical (Nasdaq: ISRG)+
announced today that it received FDA clearance for new software advancements for its Ion endoluminal robot.

Xenocor Awarded U.S. Patent for Breakthrough in Medical Borescope Technology
SALT LAKE CITY, Oct. 7, 2025 /PRNewswire/ — Xenocor, Inc., a leader in single-use surgical visualization, proudly announces the issuance of U.S. Patent No. 12,429,685 B2 for its innovative medical borescope and tip assembly technology. This milestone reinforces Xenocor’s commitment to advancing safe, efficient, and accessible minimally invasive procedures through disposable and digitally optimized laparoscopic tools.
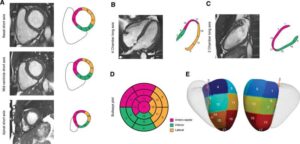
First 3D genetic mapping of the heart uncovers genes implicated in sudden death
A major cardiovascular risk factor is thickening of the heart walls (hypertrophy), which can result from high blood pressure—but is also linked to inherited diseases of the heart which can lead to sudden death.

Novel blood test for chronic fatigue achieves 96% accuracy
Scientists at the University of East Anglia and Oxford Biodynamics have developed a high accuracy blood test to diagnose chronic fatigue syndrome, also known as myalgic encephalomyelitis (ME/CFS).
