MedTech News
.................... by Andrew Celentano
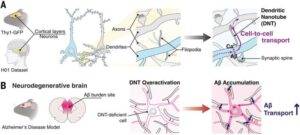
Scientists identify a new dendritic nanotubular network in the brain that may contribute to Alzheimer’s disease
Neurons in the brain communicate with each other through synapses—connection points that allow the passage of electrical and chemical signals. In non-neuronal cells, direct cell-to-cell connections have been found to occur with the assistance of nanotube structures

Leo Cancer Care Introduces Grace Upright Photon Therapy System
Grace will deliver conventional X-ray radiation therapy with patients positioned upright
CT scan changes over one year predict outcomes in fibrotic lung disease
Researchers at National Jewish Health have shown that subtle increases in lung scarring, detected by an artificial intelligence-based tool on CT scans taken one year apart, are associated with disease progression and survival in patients with fibrotic interstitial lung disease.

GlucoSet wins FDA breakthrough nod for glucose biosensor tech
GlucoSet announced today on social media that it received FDA breakthrough device designation for its glucose monitoring technology.

Gnosis for Her Unveils First-of-Its-Kind Mobile Breast CT Care Unit at More Than Pink Walk
NEWPORT BEACH, Calif., Oct. 3, 2025 /PRNewswire/ — Gnosis for Her, a mobile breast health initiative redefining comfort and access in women’s breast imaging, made its national debut this past weekend at Susan G. Komen’s More Than Pink Walk at Fashion Island. More than 6,000 breast cancer survivors, loved ones, advocates, and supporters had the opportunity to see the Mobile Care Unit up close, tour inside, and speak with the expert team behind the service.
Novel antibiotic targets IBD—and AI predicted how it would work before scientists could prove it
Researchers at McMaster University and the Massachusetts Institute of Technology (MIT) have made two scientific breakthroughs at once: they not only discovered a brand-new antibiotic that targets inflammatory bowel diseases (IBD), but also successfully used a new type of AI to predict exactly how the drug works. To their knowledge, this is a global first for the AI.
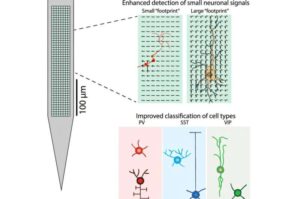
High-density brain probe reveals distinctive electrical patterns of cell types during behavior
Trying to document how single brain cells participate in networks that govern behavior is a daunting task. Brain probes called Neuropixels, which feature high-density silicon arrays, have enabled scientists to collect electrophysiological data of this nature from a variety of animals

AI chatbots often outperform doctors in diagnosis, but need safeguards to avoid overprescribing
If you’ve been to a medical appointment recently, you may have already interacted with AI. As you describe your symptoms to the doctor, they may ask your permission to use an “AI scribe” to convert audio into medical notes in real time.
