MedTech News
.................... by Andrew Celentano
FDA clears Distalmotion Dexter surgical robot for hysterectomy
Distalmotion announced today that it received FDA 510(k) clearance for the use of its Dexter robotic surgery system in hysterectomy.
Kardium reports first commercial cases with Globe PFA
Kardium announced today that it completed the first commercial procedures using its Globe pulsed field ablation (PFA) system.
Medtronic wins CE mark for left atrial appendage exclusion system
Medtronic (NYSE: MDT)+
announced today that it received CE mark approval for its Penditure LAA exclusion system.
Aidoc Receives FDA Breakthrough Device Designation for First-of-Kind AI Solution Spanning Numerous Acute Conditions in CT
NEW YORK, Sept. 30, 2025 /PRNewswire/ — Aidoc, the global leader in clinical AI, today announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the company’s novel multi-triage solution that flags a wide array of life-threatening, time-sensitive medical conditions, all within a single workflow. Built on CARE™, the first clinical-grade foundation model in healthcare with FDA cleared solutions, and deployed through Aidoc’s aiOS™ platform, the solution is designed to help care teams attend to high–risk cases faster and more consistently across the health system.

The Avive Connect AED® Approved for Use on Aircraft, Bringing the Market’s Smallest AED to the Skies
SAN FRANCISCO, Sept. 30, 2025 /PRNewswire/ — Avive Solutions, Inc. is proud to announce a significant advancement in public safety and emergency preparedness: the smallest, lightest AED on the market, the Avive Connect AED®, is safe and effective for use on aircrafts. Following rigorous testing and evaluation, this clearance confirms that the device is safe and effective for use on aircraft, enabling a new standard of emergency response at 30,000 feet.

AI at the eyelid: Glasses that track health through your blinks
Penn researchers have developed an AI-powered device that turns ordinary glasses into a smart, energy-efficient health monitor by watching you blink.
Meet your worm avatar: How microscopic worms are helping find new drugs for rare diseases
New research from the MRC Laboratory of Medical Sciences (LMS) provides a powerful, scalable method for finding treatments for rare genetic diseases using tiny, transparent worms.
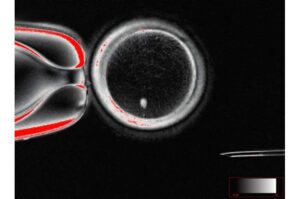
Proof-of-concept study creates functional eggs from human skin cells to treat infertility
Researchers at Oregon Health & Science University have accomplished a unique proof of concept to treat infertility by turning skin cells into eggs capable of producing early human embryos. The research is published in Nature Communications.
