MedTech News
.................... by Andrew Celentano
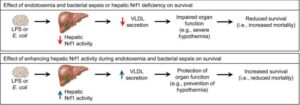
Researchers identify new method to protect against sepsis
A team of University of Saskatchewan (USask) researchers have identified a pathway to help the human body defend itself against sepsis—a life-threatening condition caused by the body’s inappropriate response to an infection.
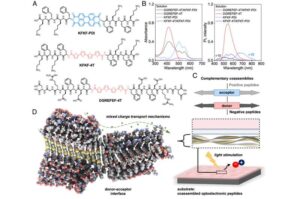
Engineers develop technology that stimulates heart cells with light
In a new study, University of California, Irvine chemical and biomolecular engineering researchers report the creation of biomolecules that can help grow light-sensitive heart muscle cells in the laboratory. The development enables a biotechnology that could deliver light-triggered signals to the heart, improving its function, without requiring genetic modifications or invasive procedures.
Machine-learning tool gives doctors a more detailed 3D picture of fetal health
MIT CSAIL researchers developed a tool that can model the shape and movements of fetuses in 3D, potentially assisting doctors in finding abnormalities and making diagnoses.
Merit Medical Embosphere Microspheres Achieve CE Mark for Genicular Artery Embolisation
Expanded indication provides patients with knee osteoarthritis with the benefits of Embosphere’s consistent, predictable, and effective clinical results

Novocure earns Japanese approval for tumor-treating fields
Novocure (Nasdaq:NVCR) announced today that it received regulatory approval in Japan for its Optune Lua wearable device.

J&J’s Shockwave launches Javelin IVL catheter in Europe
Johnson & Johnson MedTech‘s Shockwave Medical announced today that it launched its Javelin peripheral IVL catheter in Europe.

Sunsred Launches FDA-cleared Red Light Therapy Devices for Wellness Brands Worldwide
SHENZHEN, China, Sept. 15, 2025 /PRNewswire/ — Shenzhen Sunsred Technology Co., Ltd. is shaking things up in the wellness tech world with its latest lineup of red light therapy devices.

Pulse wins IDE approval
Pulse Biosciences will study PFA in cardiac surgery. Meanwhile, Galvanize Therapeutics also named a new CEO, and PFA pioneer Steven Mickelsen has launched another company.
