MedTech News
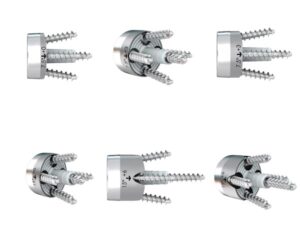
FX Shoulder Solutions, Inc. Receives FDA 510k Clearance for Full-Wedge Augmented Glenoid Baseplates
ADDISON, Texas , May 16, 2024 /PRNewswire/ — FX received 510k clearance for its full-wedge augmented glenoid baseplates. The newly cleared baseplates bring 6 new options to the previously cleared portfolio. There are now a combined total of 18 glenoid baseplate options to the market to address a variety of surgeon needs. Augmented glenoid baseplate options have continued to become a growing solution for surgeons to address bone loss, defects, or complicated morphologies of the glenoid.

FDA Grants EUA to Wondfo’s WELLlife™ COVID-19/Influenza A&B Test Supported by DCN Dx Clinical Research
CARLSBAD, Calif., May 15, 2024 /PRNewswire/ — DCN Dx, a leading contract research organization for in vitro diagnostics, today acknowledges the FDA’s Emergency Use Authorization (EUA) of the WELLlife™ COVID-19/Influenza A&B Test, developed by Wondfo USA. This important authorization will enable healthcare professionals to differentiate rapidly between COVID-19 and influenza infections, enhancing patient care at the point-of-care.
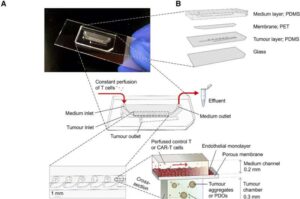
Tumor tissue on a chip: New possibilities for cell therapies and personalized medicine
How do tumors react to a certain therapeutic approach? Knowing this before the start of a therapy would be of enormous value for people suffering from cancer as well as for the doctors treating them.

Repurposed beer yeast may offer a cost-effective way to remove lead from water
A filter made from yeast encapsulated in hydrogels can quickly absorb lead as water flows through it.

Women in U.S. Can Now Collect Their Own Sample for Cervical Cancer Screening
The approval allows women to self-collect vaginal specimens for HPV testing in a health care setting, which could include non-traditional locations such as a retail pharmacy or mobile clinic.
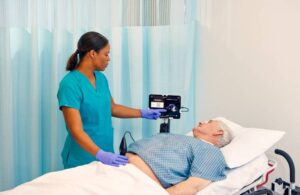
Butterfly Network launches portable ultrasound for bladder scans
Butterfly Network (NYSE:BFLY) announced today that it launched its first specialty product, the iQ+ Bladder portable ultrasound.
Atraverse wins FDA nod for left-heart access device
Atraverse Medical announced today that the FDA cleared its Hotwire radiofrequency guidewire left-heart access device.
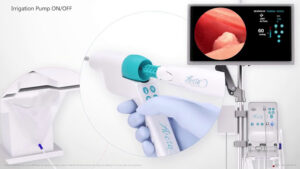
Meditrina Announces CE Mark Approval and First Multi-International Cases Performed
SAN JOSE, Calif., May 13, 2024 /PRNewswire/ — Meditrina, a leading innovator in gynecologic medical devices, announces the successful receipt of UKCA Mark, and CE Mark approval in accordance with Regulation (EU) 20147/745, for its state-of-the-art Aveta Hysteroscopy System. With this significant milestone achieved, Meditrina is thrilled to enter international markets, marking a pivotal moment in the company’s commitment to advancing women’s health globally.
