MedTech News

FDA Clears the Individualized Nomogram for Rika Plasma Donation System
LAKEWOOD, Colo., May 9, 2024 /PRNewswire/ — Terumo Blood and Cell Technologies (Terumo BCT), a medical technology company, recently received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Rika Plasma Donation System™ with the iNomi™ Nomogram. This innovation means that plasma collection volume can be determined by an individual donor’s height, weight and hematocrit level on the day they donate plasma.
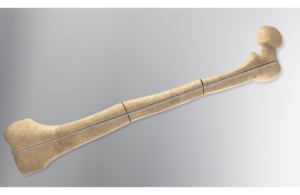
Orthofix wins FDA 510(k) clearance for Rodeo Telescopic Nail
Orthofix (Nasdaq: OFIX)+
today announced it received FDA 510(k) clearance for its Rodeo Telescopic Nail.

OrthoXel wins FDA clearance for hip fracture nail
OrthoXel announced that it received FDA 510(k) clearance for its Vertex hip fracture nail (HFN) for fracture fixation.
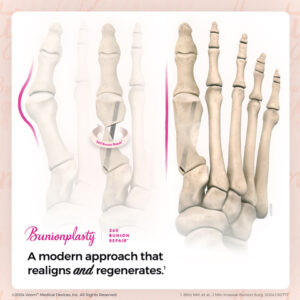
Voom™ Medical Devices’ Bunionplasty® 360 Bunion Repair™ Solution Offers Modern Alternative to Traditional 3D Approaches
The company is pleased to report a rapidly accelerating interest in its proprietary, minimally invasive Bunionplasty® 360 Bunion Repair™ solution
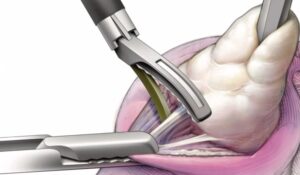
Freyja HealthCare’s VereSee(TM) Device Receives 510(k) Clearance, the First of Many Products
Freyja Healthcare Brings First-of-Its-Kind 2mm Abdominal-Access Device to Laparoscopic Surgery to Further Innovation in Fast-Growing Women’s Health Market
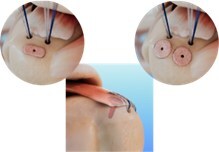
Tetrous Announces First Surgical Procedures Using EnFix Tac™ Demineralized Bone Fiber Implants to Enhance Enthesis Healing in Rotator Cuff Repair
LOS ANGELES, May 8, 2024 /PRNewswire/ — TETROUS, INC., a leading regenerative medicine company today announced that it has completed the first surgical cases using EnFix TAC™, its latest product that expands its line of EnFix™ implants to cover every surgical technique for rotator cuff repair.

4C Medical wins dual FDA breakthrough nods for mitral valve replacement device
4C Medical Technologies announced today that the FDA granted breakthrough device designation for its AltaValve system.

Outset Medical gets FDA nod for dialysis accessory months after halting sales
Outset paused shipments of its TabloCart with prefiltration last year after receiving a warning letter from the FDA.
