MedTech News
.................... by Andrew Celentano
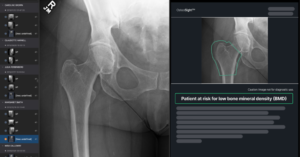
Naitive receives FDA clearance for OsteoSight
Naitive Technologies, a medical technology company developing AI-driven software to reimagine orthopedic care has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its flagship product, OsteoSight. OsteoSight enables opportunistic assessment of bone mineral density (BMD) using standard X-rays acquired to investigate other clinical concerns, such as pain associated with arthritis or a fall.

New RNA tool to advance cancer and infectious disease research and treatment
Advance from SMART will help to better identify disease markers and develop targeted therapies and personalized treatment for diseases such as cancer and antibiotic-resistant infection.
New antibody cocktail shows promise for treating multiple strains of flu
While vaccines can be very effective for preventing viruses, like the influenza A virus (IAV), they are often strain-specific and prone to viral escape mutations. IAV alone is responsible for around 500,000 deaths worldwide each year.
Customized gene-editing technology shows potential to treat lethal pediatric disease
Multisystemic smooth muscle dysfunction syndrome (MSMDS) is a rare condition associated with stroke, aortic dissection (tearing) and death in childhood. Currently, there is no effective treatment or cure for MSMDS.
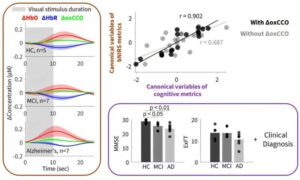
Portable light-based brain monitor shows promise for dementia diagnosis
Early and accurate diagnosis of dementia remains a major challenge. Standard approaches such as MRI and PET scans can provide valuable information about brain structure and function, but they are expensive, not always accessible, and often too expensive for repeated use.
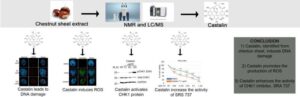
Chemotherapy plus natural extract castalin improves cancer drug effectiveness, study finds
Scientists have identified a natural extract that could help boost the effectiveness of cancer drugs. The insights gained from their study may help formulate new combination drug therapies, using precision medicine to target and cure cancer and improve patient outcomes.
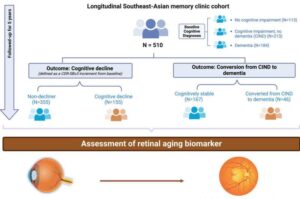
AI-powered eye scan predicts risk of cognitive decline and dementia
A new study led by researchers from the Yong Loo Lin School of Medicine at the National University of Singapore (NUS Medicine) has demonstrated that artificial intelligence (AI) analysis of retinal photographs can predict an individual’s risk of cognitive decline and dementia.
Teleflex enrolls first patient in dual-drug-eluting device study of coronary interventions for diabetes
Teleflex (NYSE:TFX) announced today that it enrolled the first patient in its DUBSTENT DIABETES trial.
