MedTech News
.................... by Andrew Celentano

Slingshot Tongues of Chameleons and Salamanders Could Transform Tech in Medicine and Space
Learn more about the biological mechanism behind the tongue movement of chameleons and salamanders, which could contribute to critical technological breakthroughs.

Mendaera reports first procedures with handheld surgical robotic system
Mendaera today announced the world’s first procedures performed with its Focalist handheld robotic surgery system.

Tasso-SNBL Secures Japanese Certification for Self-Collection Blood Kit
Breakthrough approval supports home-based testing and strengthens access to care
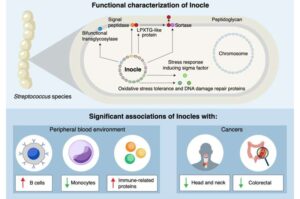
Giant DNA discovered in people’s mouths could impact oral health, immunity and even cancer risk
Researchers, including those at the University of Tokyo, have made a surprising discovery hiding in people’s mouths: Inocles, giant DNA elements that had previously escaped detection. These appear to play a central role in helping bacteria adapt to the constantly changing environment of the mouth.

A ‘universal’ therapy against the seasonal flu? Antibody cocktail targets virus weak spot
An unusual therapy developed at The Jackson Laboratory (JAX) could change the way the world fights influenza, one of the deadliest infectious diseases. In a new study in Science Advances, researchers report that a cocktail of antibodies protected mice—including those with weakened immune systems—from nearly every strain of influenza tested, including avian and swine variants that pose pandemic threats.
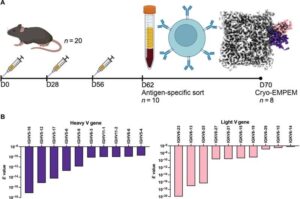
AI model helps identify therapeutic antibodies to boost pandemic preparedness
Scientists at Scripps Research have developed a novel method that uses artificial intelligence (AI) and advanced imaging techniques to more accurately and efficiently identify therapeutic antibodies to treat infectious diseases.
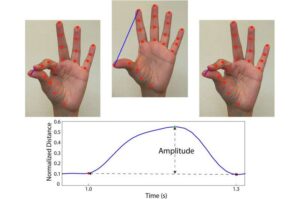
AI detects subtle movement changes in finger-tapping videos, revealing hidden Parkinson’s signs
Early detection of even the slightest motor function changes can be critical to slowing the progression of Parkinson’s disease. Yet these subtle signs often go unnoticed. Now, UF researcher Diego L. Guarín, Ph.D., is harnessing AI to spot these subtle changes from video recordings before clinical symptoms become evident to the clinician’s eyes.
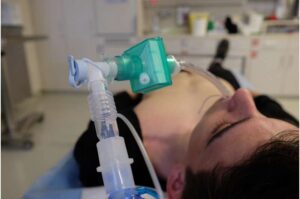
Soft robot intubation device, designed specifically for non-expert users, could save lives
Maintaining an open airway is a critical priority in emergency medicine. Without the flow of oxygen, other emergency interventions can become ineffective at saving the patient’s life. However, creating this airway through endotracheal intubation is a difficult task for highly trained individuals and under the best of circumstances.
