MedTech News
.................... by Andrew Celentano

Biozen Presents Calibration-Free, Cuffless Blood Pressure Technology at AHA 2025
OKLAHOMA CITY, Sept. 4, 2025 /PRNewswire/ — Biozen, LLC a digital health innovator integrating advanced biosensors with proprietary algorithms, will present its cuffless blood pressure technology at the American Heart Association (AHA) Hypertension Scientific Sessions on Sept. 5 at 9:00 a.m. in Baltimore, Maryland (Poster Session 2, Poster #FR443).
Ultrasound AI Secures New U.S. Patent for AI-Driven Clinical Value Determination, Expanding Global Patent
DENVER, Sept. 4, 2025 /PRNewswire/ — Ultrasound AI™, Inc., a pioneer in artificial intelligence for medical imaging, today announced that the United States Patent and Trademark Office has issued U.S. Patent No. 12,369,883, “Artificial Intelligence System for Determining Clinical Values through Medical Imaging.” The patent protects the company’s proprietary system for determining current or future clinical or laboratory values directly from non-invasive medical images such as ultrasound.
BVI unveils dual cataract, vitreoretinal surgical platform
Ophthalmic device company BVI Medical unveiled its new Virtuoso phaco-vitrectomy surgical platform.
Ypsomed gets FDA nod for digital add-on for autoinjectors
Ypsomed announced that the FDA granted 510(k) clearance for SmartPilot, a digital connectivity add-on for its Ypsomate autoinjectors.

Roche wins CE mark for Susvimo drug-eluting eye implant
Roche announced today that it received CE mark approval for its port delivery platform containing its Susvimo therapeutic.
Intense light therapy may lower risk of myocardial injuries after non-cardiac surgery
Intense light therapy after surgery can increase a critical protein that protects heart tissue while lowering levels of troponin, a protein indicating heart damage that’s linked to higher mortality in patients undergoing non-cardiac surgery, according to a study by researchers at CU Anschutz.
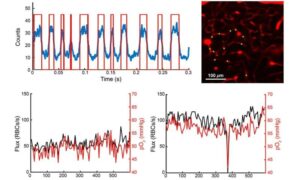
High-resolution imaging uncovers hidden risks of brain capillary stalls
The brain depends on a constant supply of oxygen, delivered through an intricate network of tiny blood vessels. Unlike other organs, it has little energy stored and is particularly sensitive to interruptions in blood flow

Philips launches smart telemetry platform for cardiac monitoring
Philips (NYSE: PHG)+ today announced the introduction of a new telemetry platform aiming to address critical challenges in healthcare.
