MedTech News
.................... by Andrew Celentano
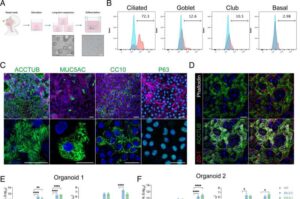
Organoid-based platform enables precise evaluation of antibody and vaccine efficacy
A research team has successfully developed the world’s first nasal organoid-based SARS-CoV-2 neutralizing antibody evaluation platform.
Scientists develop off-the-shelf immunotherapy for metastatic kidney cancer
UCLA researchers have developed a new kind of immunotherapy that uses specially engineered immune cells equipped with built-in weapons to attack kidney cancer tumors and reprogram their protective environment—all without the need to customize treatment for each individual patient.
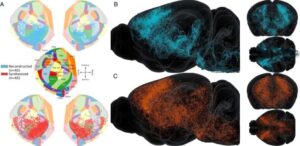
Scientists create realistic brain-wide connection maps through digital modeling
EPFL researchers have developed a powerful method to generate brain-wide, biologically realistic wiring maps of the mouse brain. Their approach bridges experimental data with mathematical and computational modeling to simulate how neurons connect across the entire brain.
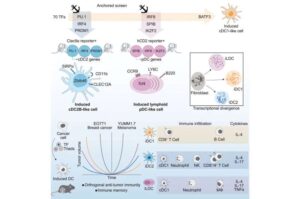
Unlocking the immune system: Cellular ‘toolkit’ could reprogram cells for cancer therapy
An international team led by researchers at Lund University in Sweden has identified the molecular tools needed to reprogram ordinary cells into specialized immune cells.
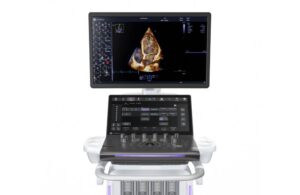
GE HealthCare launches new AI-powered cardiovascular ultrasound system
GE HealthCare (Nasdaq: GEHC)+
today announced the launch of Vivid Pioneer, its newest, most advanced cardiovascular ultrasound system.
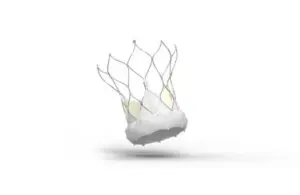
Abbott wins expanded CE mark for Navitor TAVI system, reports updated TEER guidelines
Abbott (NYSE: ABT)+
announced today that it received CE mark for an expanded indication for its Navitor TAVI system.
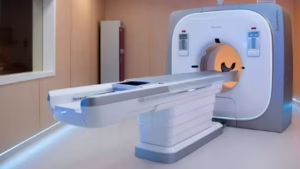
Neusoft Medical introduces NeuViz P10 PCCT system in China
The NeuViz P10 uses a cadmium zinc telluride detector to directly convert X-ray photons, thereby eliminating light conversion.

Immune cell therapy for advanced head and neck cancer helps stabilize disease
A multi-institutional clinical trial conducted at the UNC Lineberger Comprehensive Cancer Center and 21 other U.S. sites found that a single administration of autologous tumor-infiltrating lymphocyte (TIL) cell therapy helped stabilize metastatic head and neck squamous cell carcinoma (HNSCC) in some patients. This finding is significant, as many of these patients had previously undergone multiple treatments without success.
