MedTech News
.................... by Andrew Celentano
Gene therapy restores functionality in nonhuman primates after heart attacks
Biomedical engineers at Duke University have successfully conducted experiments to treat damage caused by heart attacks in nonhuman primates using gene therapy for the first time.
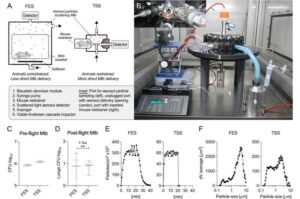
New ‘cough simulator’ mimics tuberculosis transmission with unprecedented accuracy
Tuberculosis has been a scourge upon humanity throughout history. In killing more than a million each year worldwide, it remains the leading cause of death from a single infectious pathogen.
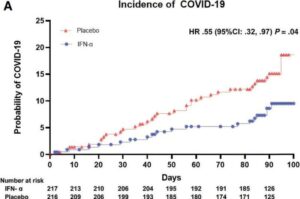
Nasal spray reduces COVID-19 risk in cancer patients, clinical trial shows
A world-first clinical trial has found that a simple daily nasal spray can significantly reduce the risk of COVID-19 in cancer patients, offering a potential new tool to protect vulnerable people from the virus.

Faster diagnostic method can detect sepsis in hours instead of days
A new diagnostic method would confirm sepsis infections earlier, cutting critical hours in the “race against time” to save patients’ lives.

Novel therapy for pet cats with head and neck cancers could help humans
Researchers have reported results from the first-ever clinical trial of a new class of targeted therapy in pet cats with head and neck squamous cell carcinoma (HNSCC)—a cancer which is notoriously deadly and difficult to treat.
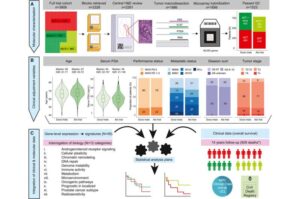
Molecular test helps tailor chemotherapy for advanced prostate cancer patients
Testing the molecular profile of tumors identifies which patients with advanced prostate cancer are more likely to benefit from chemotherapy and live longer, sparing patients less likely to benefit from unpleasant side effects, according to a new study led by UCL researchers.

AI and lab tests combine to predict disease risk from rare genetic variants
When genetic testing reveals a rare DNA mutation, doctors and patients are frequently left in the dark about what it actually means. Now, researchers at the Icahn School of Medicine at Mount Sinai have developed a powerful new way to determine whether a patient with a mutation is likely to actually develop disease, a concept known in genetics as penetrance.
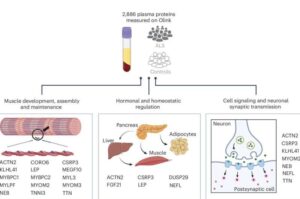
Novel blood test for ALS can detect early signs years before symptoms appear
Current ALS diagnosis relies on neurological evaluations and the presence of symptoms; at present, there is no definitive diagnostic test.
