MedTech News
.................... by Andrew Celentano
Blood pressure calculator promises more precise medication choices for millions
A first-of-its-kind Blood Pressure Treatment Efficacy Calculator built on data from nearly 500 randomized clinical trials in over 100,000 people allows doctors to see how much different medications are likely to lower blood pressure.

MIT researchers develop AI tool to improve flu vaccine strain selection
VaxSeer uses machine learning to predict virus evolution and antigenicity, aiming to make vaccine selection more accurate and less reliant on guesswork.
Blood test able to detect ALS up to a decade before symptoms start
Current ALS diagnosis relies on neurological evaluations and the presence of symptoms, with no definitive diagnostic test currently available.
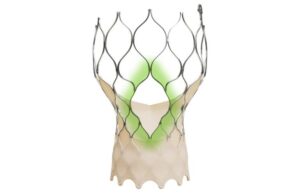
Medtronic Evolut wins FDA nod for expanded redo TAVR indication
Medtronic (NYSE: MDT)+
announced today that it received FDA approval for the expanded redo-TAVR indication of its Evolut system.
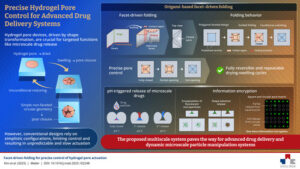
SEOULTECH Researchers Develop Smarter, More Controllable Hydrogel Pores
In a recent study, researchers introduced an origami-inspired “facet-driven folding” strategy using polygonal hydrogel pores to deliver highly controlled, programmable actuation, opening new possibilities for selective drug delivery and information encryption.
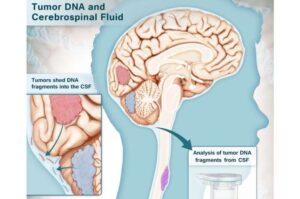
Test detects brain cancers in cerebrospinal fluid with high accuracy
A novel, multi-analyte test developed by researchers at the Johns Hopkins Kimmel Cancer Center, its Ludwig Center and the Johns Hopkins Department of Neurosurgery can accurately identify brain cancers using small samples of cerebrospinal fluid (CSF), offering a promising new tool to guide clinical decision-making.
Gene therapy leads to improved quality of life in patients with sickle cell disease and beta thalassemia
Treatment with exagamglogene autotemcel (exa-cel) led to robust and sustained improvements in quality of life for patients with severe sickle cell disease (SCD) or transfusion-dependent beta thalassemia, according to two studies published in Blood Advances.
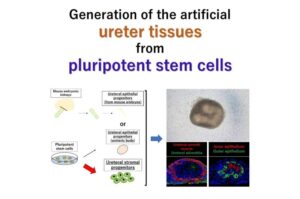
Functional ureter tissue created from stem cells paves way for transplantable kidneys
Scientists at Kumamoto University have made a major breakthrough in regenerative medicine by successfully creating functional ureter tissue—organoids resembling the urinary tract—from pluripotent stem cells.
